Customized quality control for medical device manufacturers
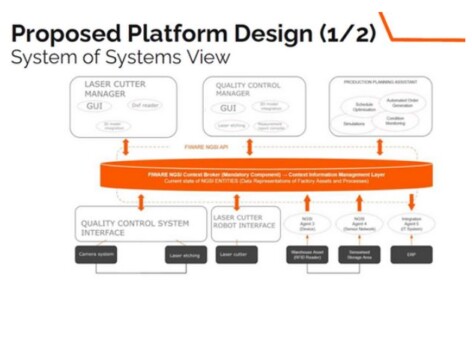
Creating a flexible, but reliable production line is an important aspiration for all manufacturers This is especially the case for the manufacturers in the medical technology sector, since the quality standards are high (EU 745 2017 ( and ISO 13485 which can be challenging to maintain for SMEs because they tend to manufacture multiple products in a smaller scale.
Contact
Krakow Technology Park sp. z o.o.
ul. Podole 60
30-394 Kraków, Poland